



The precious metals are known for their beauty and their price. Gold has a distinctive yellow gleam; silver looks intensely white, and all of the noble metals are so called because they resist oxidation and other chemical reactions.
  |
  |
  |
  |
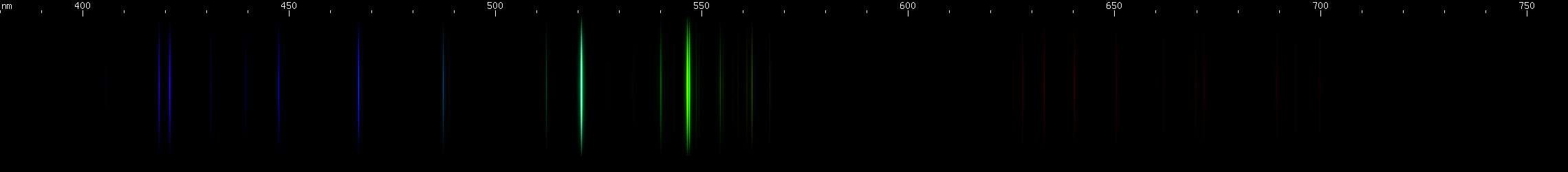
Silver: Green lines dominate the silver spectrum: grass green near 547nm (actually two lines), and more of an emerald green near 521nm. A deep violet line is also apparent near 405nm. The spacing of these three bright zones recalls mercury or krypton, however none of silver's bright lines are yellow. Both silver and copper produce a bright green spark, but their green lines form very different patterns.
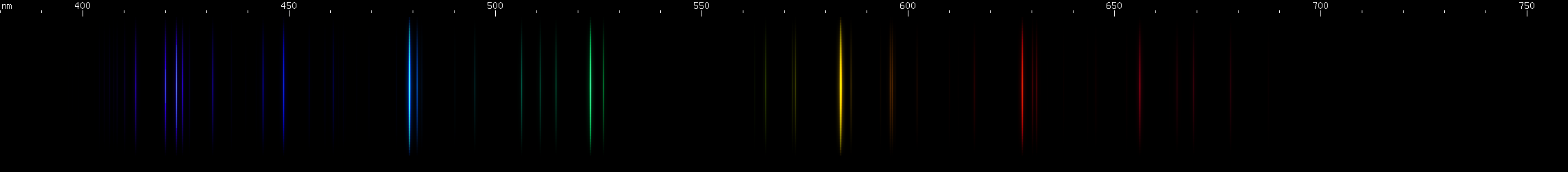
Gold: The most visually striking feature is a greenish blue line at 479.3nm. Its hue is distinctive enough that not many elements emit a bright line this color on its own without any strong neighboring lines. Gold's bright yellow (584nm), red (628nm), and emerald green (523nm) lines form a two pair pattern with its blue line: a bright and brighter pair, then an evenly matched pair. In the green region, there are some lines that vaguely resemble copper, and there is a pattern of blue and violet lines that seems to go dim, bright, dim, bright, with a specific, easily recognizable, almost repeating, pattern of spacing.
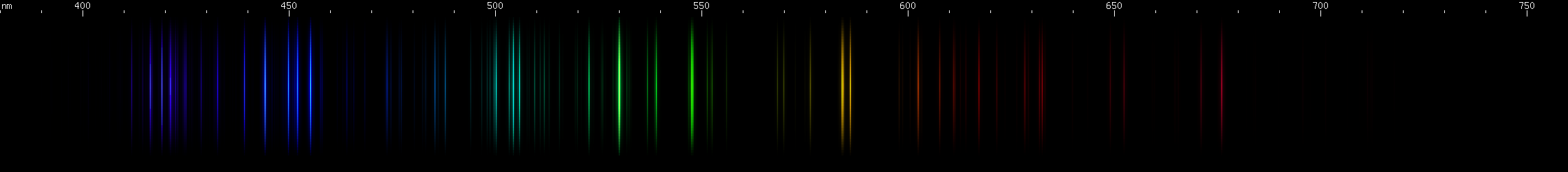
Platinum: This one has a pattern of lines that, while not simple, can be recognized if you are familiar with it. The bright lines are: three close together indigo; teal (three very close together, tends to look like just one line); four or five green; one or two yellow (one of which is a multiplet anyway). Additional lines can be seen in the violet and azure, and a pattern of lines that go orange-red-red, red, red.
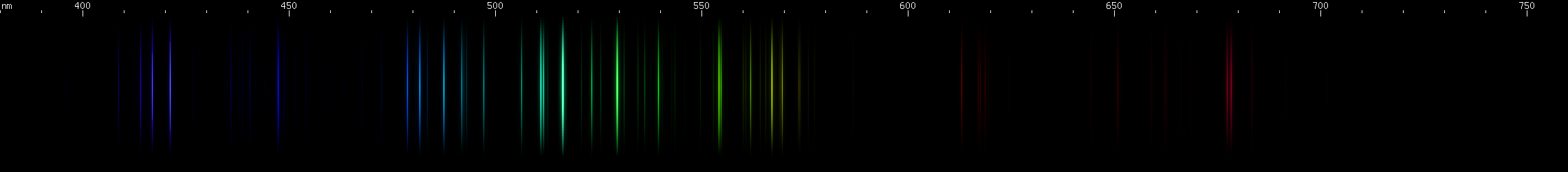
Palladium: Resembles platinum at first glance, but has some key differences. Has only one visually observable red line, actually three very close together lines near 688nm, compared to platinum's series of well separated orange and red lines. There is no discrete strong yellow line for palladium, although the sequence of green lines begins at about 570nm greenish yellow. Greens appear brighter towards shorter wavelengths, vs. platinum's greens which are closer together and more even in brightness. The intensities of the blue lines give a different "shape", i.e. brighter at shorter wavelengths rather than brightest in the middle. The violet line is considerably brighter in palladium than platinum, relative to the blue lines.
Gold is a lookalike for another metal, rhenium:
  |
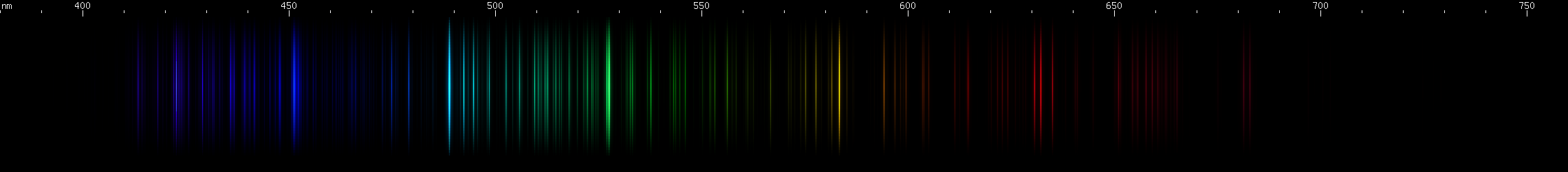  |
The most obvious difference is the color of the blue-green line: in gold it's fairly blue, 479nm or a beautiful teal-azure. In rhenium, the blue-green line is noticeably greener, about 489nm. There's another difference in the red region: gold has a noticeable secondary red line closer to its main red line than main red is to yellow, whereas rhenium's secondary red line is more evenly spaced and harder to see. You can compare then here to find more differences.
Next: Semimetals