

Let's start with the two spectra that everyone learns first, and the one that is the most distinctive.
  |
  |
  |
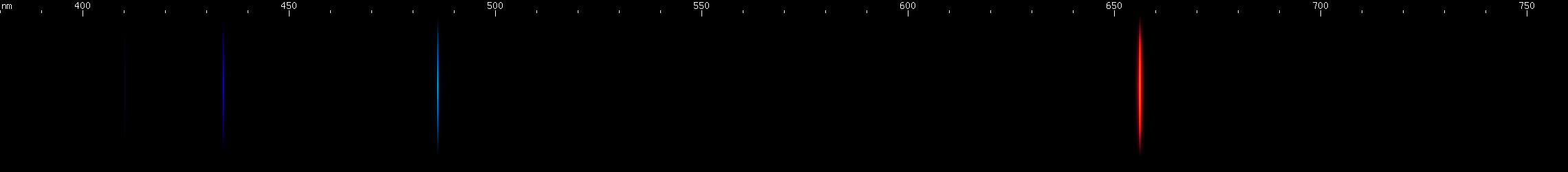
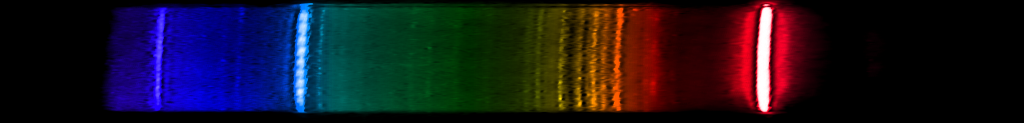
Hydrogen: It's a familiar sequence of lines from chemistry and astronomy textbooks. The most abundant element in the universe emits one bright red line, one somewhat weaker greenish-blue, and a series of violet and ultraviolet lines that are progressively weaker and closer together. This is the Balmer series, one of many series that hydrogen emits, and the only one we can see (most of) with our eyes. It's a distinctive pattern, and while a few element spectra superficially resemble hydrogen, none have that pattern of decreasing space.
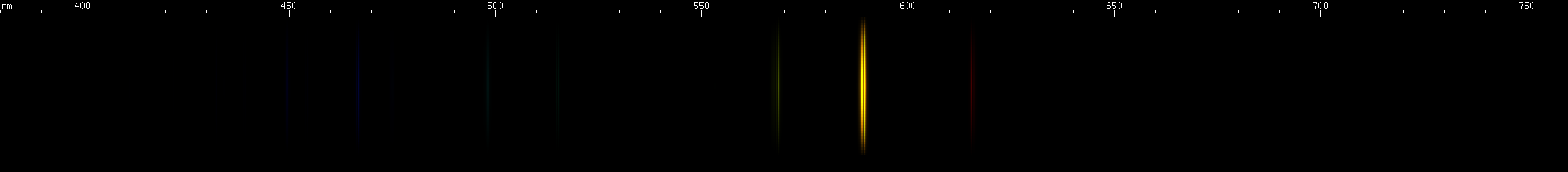
Sodium: A pair of intense orange-yellow lines so close together that they are difficult to resolve and usually look like a single line. Sodium gets in everything. I had to clean this amber doublet out of almost every spectrum photo I took for this site! (Not the gases though.) Human skin always bears a thin layer of sweat and oils, and this layer deposits salt on everything we touch. Once there, it adds sodium lines to a spark spectrum. Most of the time, only the amber lines will be visible, but in lamps that energize sodium vapor at pressure, the pattern of red, chartreuse, and blue-green to violet can be seen as shown in the photo. If you look closely, you can see that the red and chartreuse lines are farther apart than the two blue-green lines, which then are farther apart than the two blue lines, and so on. These are in fact two converging series, similar to the Balmer series of hydrogen! Their alpha lines are located in the infrared. In fact, all atomic spectra are made of series like these, due to the way atomic energy levels get progressively closer together towards a limit. Even the amber doublet is the alpha of its own series, with the remaining lines located in the ultraviolet. And the limit that a neutral atom's energy levels approach, the energy level of the hypothetical shell with quantum number n = infinity, is none other than the atom's first ionization energy.
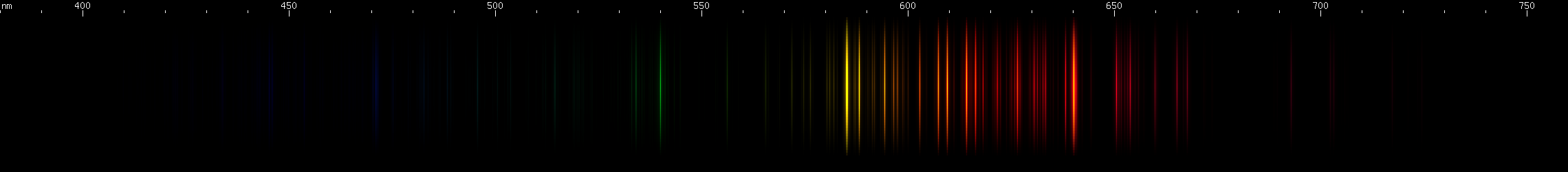
Neon: The most commonly used gas for discharge tubes. (Only mercury and sodium are used more, but those are technically vapors, not gases per se.) Nothing else makes this pattern of yellow, orange, and red lines. Look for the bright yellow and bright red, with the two clusters of orange almost exactly between, and various moderately strong lines all in between and continuing into the far-red region. Once you're familiar with the pattern, it's unmistakable, and you'll always recognize it immediately even in mixtures. (In case you're wondering - the bright red, orange, and yellow lines of neon are also part of their respective series. Most of them are "alpha" to their series, with the remaining lines in the ultraviolet region. For this reason, neon emits a small amount of "blacklight" UV.)
Next: Common Lighting