


Now for two of the easiest metals to recognize, and one very common metal that actually has some imitators:
  |
  |
  |
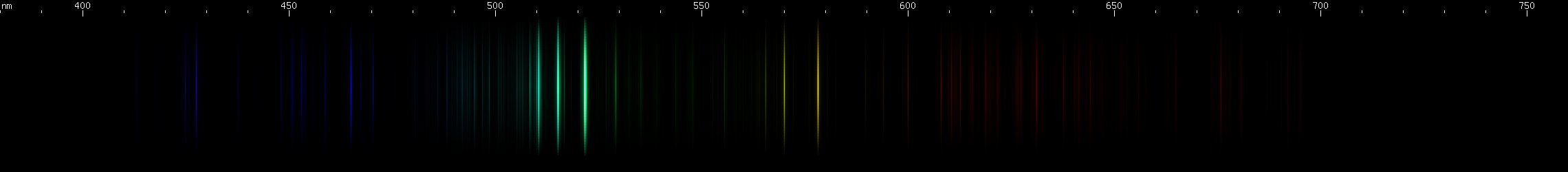
Copper: Copper is known as a red (really brownish pink) metal that makes green flames, green sparks, and green oxide. The last of these is a coincidence, but copper does indeed emit a distinctive grouping of green lines. Visually it appears to be three lines, although more lines can be resolved with sensitive equipment. Additionally, two greenish-yellow lines complete an easily recognizable pattern, which is then rounded out by various fainter blue and red lines.
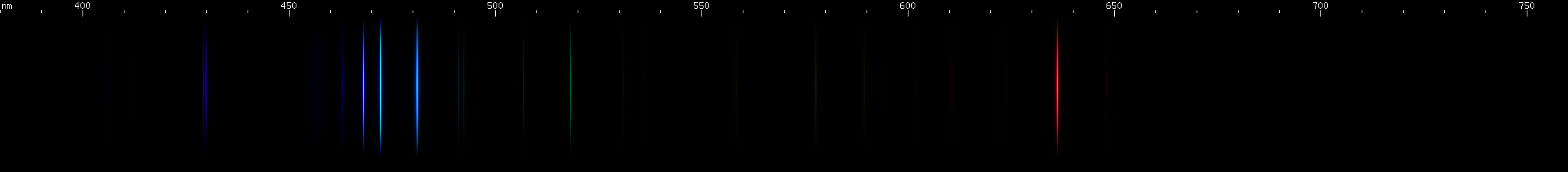
Zinc: Zinc sorta looks like someone took copper's spectrum and spread it out more. Like copper, zinc has a trio of beautiful bright lines, except they're azure instead of emerald. Zinc's red line is as distinctive as copper's yellow lines. The photo actually depicts the spark spectrum from two galvanized bolts. Consequently, some very faint iron emission may be visible. The important thing for the sake of zinc is the pattern of blue and red.
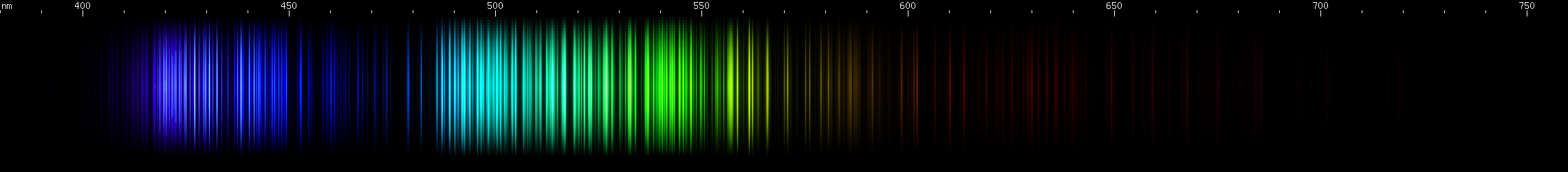
Iron: Iron is what I'll call a darned many lined spectrum dominated by greens and blues, or DMLSDGB. Nah, too many letters. Okay, how about just darned multi line. DML. Several elements are DMLs, and it can be easy to confuse them. Botany enthusiasts who observe plants in the wild complain about darned yellow composite (DYC) and darned white composite (DWC) flowers, so called because there are so many species that look similar and can be difficult to tell apart. Fortunately, there are interspecific differences between DYC and DWC plants (how else would they be resolved as different species?), and similarly, there are interelemental differences between DMLs. Every element's spectrum is like a unique fingerprint, even DML elements. In the case of iron, look for the violet gap, a region around 435nm with no bright lines. Other elements that look superficially similar, lack this violet gap, or have their violet gap at some other wavelength with a different pattern of bright lines surrounding it.
Next: Lookalikes: Part I