


These look a little more similar to each other, so we'll give you hints to differentiate them easily:
  |
  |
  |
All three of these have a prominent red line past 640nm, one or more noticeable yellow-green lines, and something in the blue-violet region. Two of them have strong blue-green and deep violet. While all three have similar features, their overall look is different.
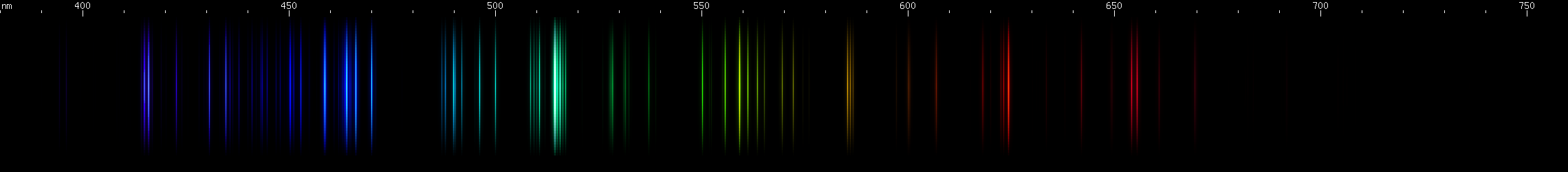
Aluminum: The bright emerald-green lines around 515nm stand out. These are from singly ionized aluminum, or Al II (see the secion on ion spectra), however aluminum is often alloyed with magnesium, which also emits lines very close to these. Other lines of Al II in the green and blue regions appear less intense further from the bright emerald-green lines. The most intense visible lines of aluminum are actually the deep violet 395nm doublet, which is a transition to ground state. Ground state is the lowest energy level of an atom or ion, and transitions that end on ground state are responsible for the strongest lines an element emits. For neutral aluminum, or Al I, the ~395nm doublet is so strong that it is usually perceptible even though the human eye is only very weakly sensitive to these wavelengths. Near the ~395nm doublet is another pair of lines at ~415nm from Al II. It is easy to confuse the two pairs when making visual observations, although they may look slightly different in hue.
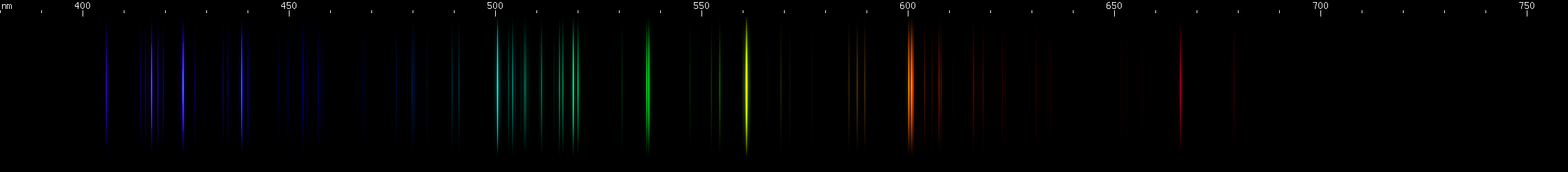
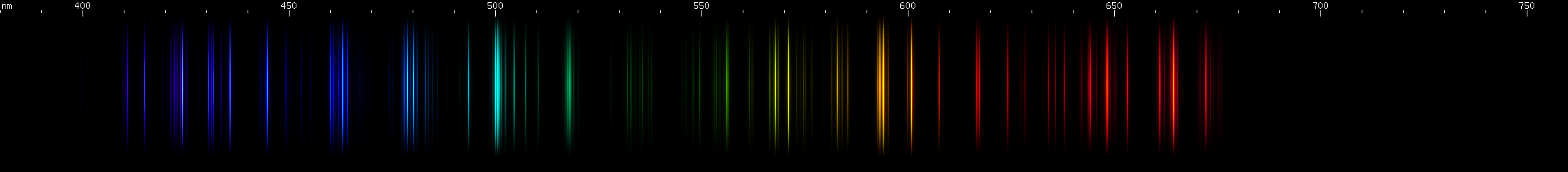
Tin: Tin's spectrum doesn't really resemble any other element. The pattern of two close together red lines, a few yellow-green, and then two close together blue lines, in an almost evenly spaced configuration, identifies tin. The yellow-green lines are flanked by yellow and purer green, and the red is accompanied by another deeper red line near 685nm.
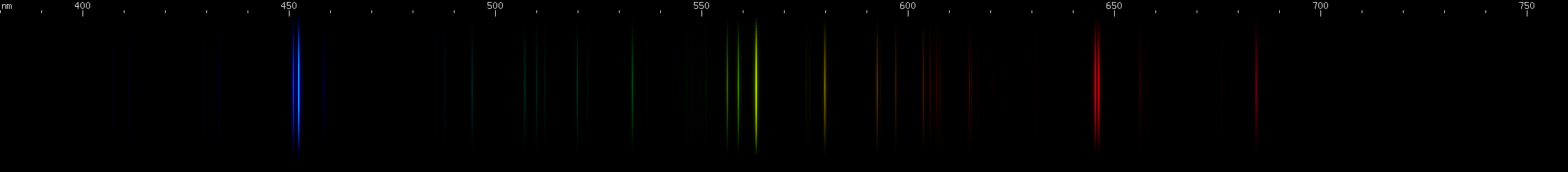
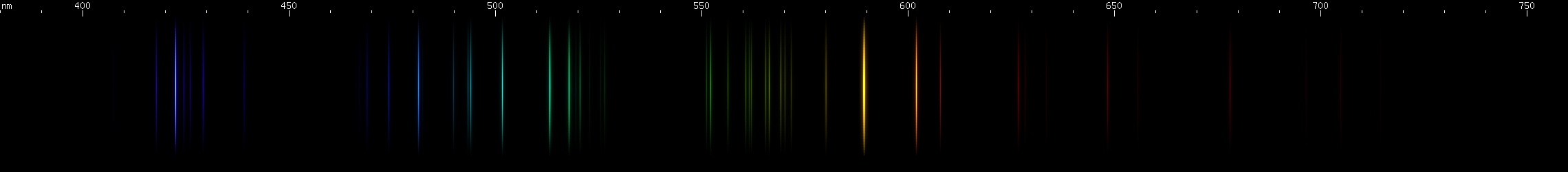
Lead: The strong lines at 406nm deep violet and 561nm yellow-green are lead's most distinguishing features. The teal grouping near 504nm also helps identify lead, along with the pattern of green lines between it and the strong yellow-green line. The strong red line is at 666nm, perhaps fittingly for such a toxic element; it stands alone, with no other prominent red lines. There is a pattern of yellow and orange lines, and a pattern of blue-violet lines.
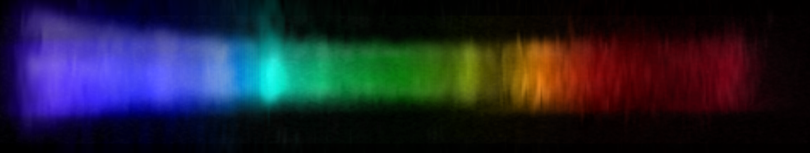
Lead and tin are often combined to make solder. The photo below depicts Sn63/Pb37 solder, the most common formulation.
 |
 |
 |
 |
 |
 |
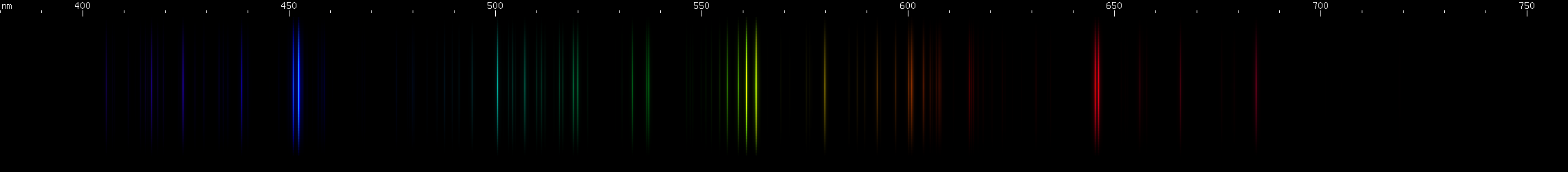
You can see that the green lines are predominantly tin, but that lead lines are obvious where tin does not have many bright lines, particularly in the violet region.
We have a few more near lookalikes for lead: one spectrum that surprisingly resembles lead very closely, and two more that have a pair of distinctive lines that resemble lead lines.
  |
  |
  |
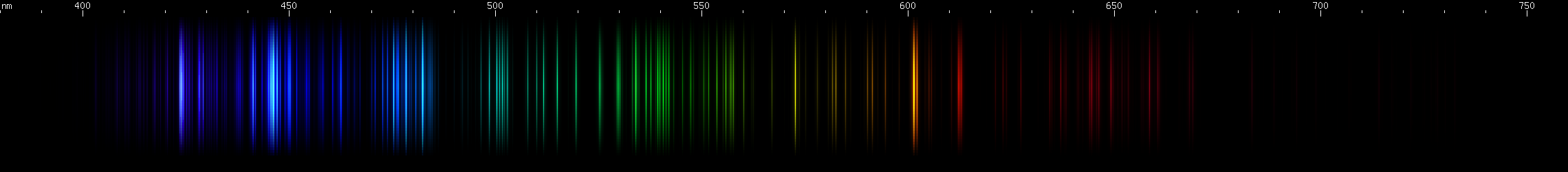  |
Lead vs. Nitrogen: The resemblance is uncanny, and at first glance it may seem impossible to tell them apart in the photos. But look at the pattern of green lines between the teal and chartreuse lines; they're different patterns for the two elements. Also, nitrogen does not have a moderately bright yellow line near its orange line like lead does; instead, there is a faint yellow that can be seen halfway between the orange and chartreuse. The pattern of orange-red lines in nitrogen is absent from lead. And while both elements have a strong deep violet component, the pattern of blue lines is very different between the two.
The nitrogen photo, by the way, is actually an air arc. While nitrogen at atmospheric pressure forms N2 molecules, in an arc such as lightning or the arc from an automotive ignition coil, these molecules are experimentally observed to dissociate, giving lines of atomic N. The contribution of oxygen is negligible, because recall that oxygen has no intense visible lines. Other gases such as argon and carbon dioxide simply do not occur at high enough concentration to appear in the photo. However, nitrogen spectrum tubes universally exhibit molecular N2 bands, as if it is somehow impossible to make an atomic nitrogen tube:

Lead vs. Germanium: Germanium lacks a strong yellow-green line, and does not have any red lines that are proportionally as strong as lead's. Where the two can be easily confused is in the yellow-orange region, where both have a strong yellow line and an almost as strong ornage line. (For lead, these are groups of close together lines.) The difference is that germanium's yellow and orange are visually its brightest lines, with one significantly brighter than the other, while for lead they are visually less important and more similar to each other in terms of brightness. Germanium differs significantly from lead in the green, blue, and violet regions, and overall its spectrum is shaped more like that of tin. That kind of resemblance can be forgiven since all three are in the same column of the periodic table, the so-called carbon family.
Lead vs. Manganese: Manganese also has a lookalike for lead's orange and yellow lines: an orange line and an orange-red line, although each one is actually a few lines very close together. For manganese these lines occur around 602nm and 613nm, giving them a much redder appearance than lead's 588nm yellow or germanium's 589nm. Also, like germanium, manganese has a stronger orange than red, compared to lead where the yellow and orange have almost equal visual brightness. Otherwise, manganese does not resemble lead at all and in fact can be easily recognized by its pattern of nearly evenly spaced orange-green-azure followed by its distinctive blue/violet pattern. Manganese's bright azure lines also resemble zinc, except closer together and without a similarly bright red line.
Next: The Most Beautiful Metals