

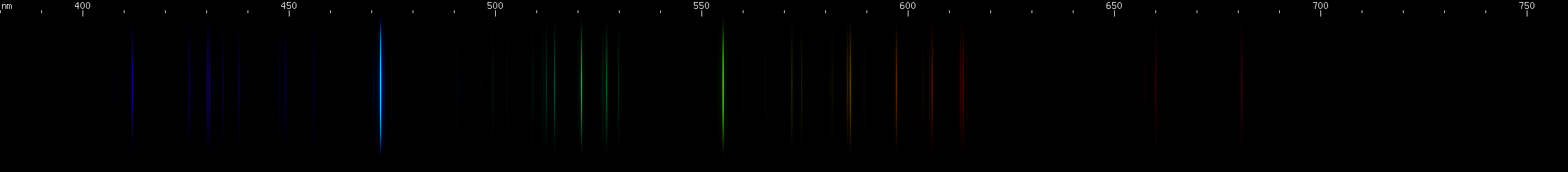

  |
  |
  |
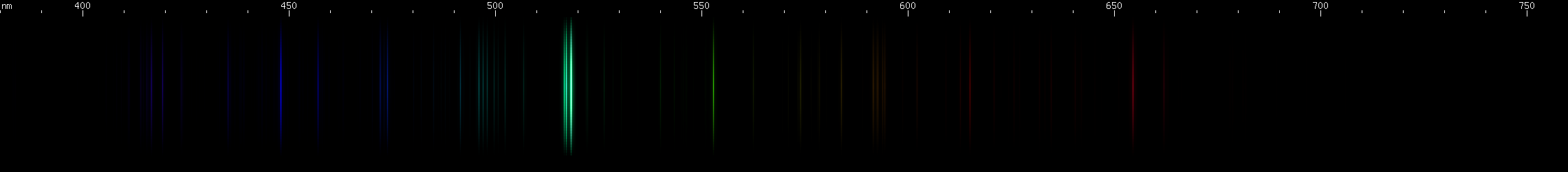
Magnesium: It is dangerous to subject magnesium to electric sparks, because of the possibility of igniting this reactive metal, however if done in small quantities, with adequate safety precautions, the color produced is stunning, and cannot be done justice on a computer monitor. Magnesium makes a vivid deep teal, dominated by its bright triplet near 517nm. Recall that aluminum has bright lines in almost the same place. Recall also the bright blue triplet of zinc, becoming a more spread out blue-green in cadmium, and then greena nd violet in mercury. Magnesium's bright green triplet is analogous to these triplets of zinc through mercury, arising from the same transition in the same part of its energy level diagram. Technically, we can say that it's a 3S (read: "triplet ess") transition, which occurs when an excited electron moves from magnesium's 3s.4s to one of its 3s.4p levels on the triplet side. Triplets occur when two unpaired electrons have the same spin. For zinc, the electrons move from 4s.5s to 4s.4p; for cadmium, 5s.6s to 5s.5p, etc.
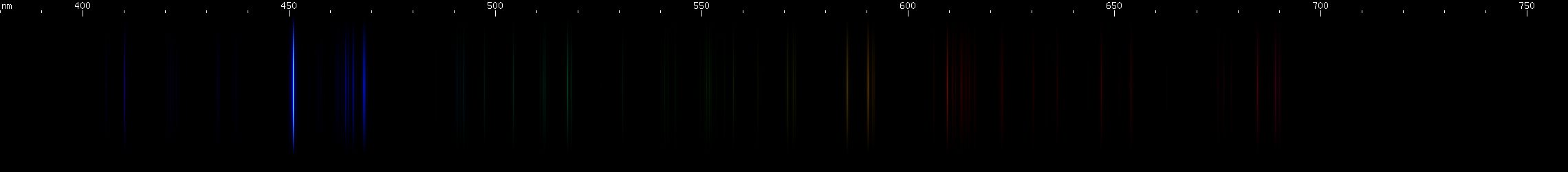
Indium: For a metal named after one of its spectral lines, it is fitting that it would produce a beautiful, deeply saturated spark. Neutral indium's ground state configuration produces two energy levels, differing by the orientation of the outer electron's spin relative to its orbit. A visible doublet occurs from that electron transitioning from an upper layer to the split ground state. One member of the doublet is in the deep violet, and occurs on the transition to the true ground state; the other member of the doublet, from the transition to the level just above ground, has a wavelength of 451.1nm which is much more easily visible to the eye than the deep violet wavelength is. The presence of a visible transition to ground state, in the neutral atom, means the visible spectrum of indium is dominated by these two lines. Better yet, neutral In I does not make any other intense visible lines; most of its lines are ultraviolet or infrared. Best of all, ionized In II has all of its strongest lines in the UV so indium, unlike its lighter relative aluminum, isn't full of bright ionization lines. Therefore, the bright In I doublet pretty much is the visible spectrum of indium.
Thallium, too, has a visible spectrum made up of essentially one bright line, and for the same reason as indium, since they are congeners, elements of the same column of the periodic table. In thallium, the equivalent to indium's 451.1nm line is shifted to 535.0nm, and the equivalent to the deep violet line occus in the UV at 377.6nm. As thallium is extremely toxic, and there really isn't anything to learn from photographing its spectrum, I have opted not to obtain a sample of thallium.
Gallium, also a congener of aluminum, indium, and thallium, has spectroscopic properties similar to indium, at wavelengths intermediate between indium and aluminum. The bright Ga I doublet occurs at 417.2nm and 403.3nm, at a deep violet wavelength but not so hard to see as aluminum's doublet. Besides this deep violet, gallium shows a pair of faint Ga I red lines and a somewhat stronger Ga II red line (633.4nm), but is still visually overwhelmingly dominated by its violet doublet, and the spark produced is strongly violet in color.
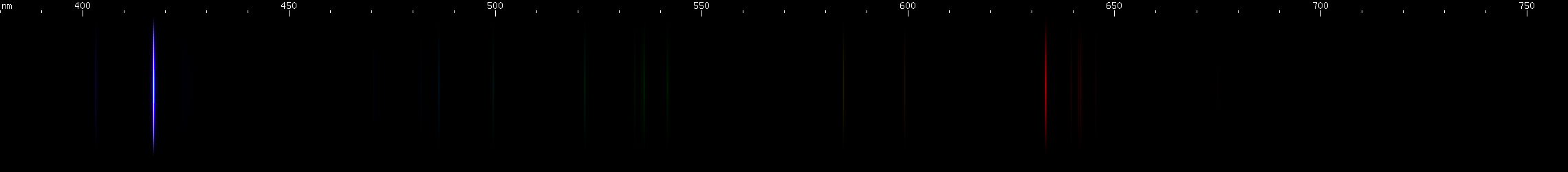

Bismuth: This is a metal with a low melting point that crystallizes easily into beautiful stairstep patterns, and then develops a colorful iridescent oxide coating. Fittingly, its spectrum is also beautiful, featuring one particularly bright blue line, in the bluer part of the azure region, at 472.3nm. This line occurs from a neutral Bi I transition, not to ground but to the next level up, so low that the transition from that level to ground generates an infrared line (875.5nm). A transition to such a low level in the neutral atom guarantees that this will be one of bismuth's most intense lines. Bismuth is easily distinguished from indium by the color of its bright blue line, closer to azure or denim blue vs. indium's eponymous indigo. It can also be distinguished by its green lines and yellow/orange lines, visually absent from indium, and by the pattern of blue-violet lines between its bright blue line and deep violet line. Bismuth's spark has nowhere near the deep saturated color as indium's, due to the presece of these moderate intensity visible lines.
Next: Alkaline Earth Metals