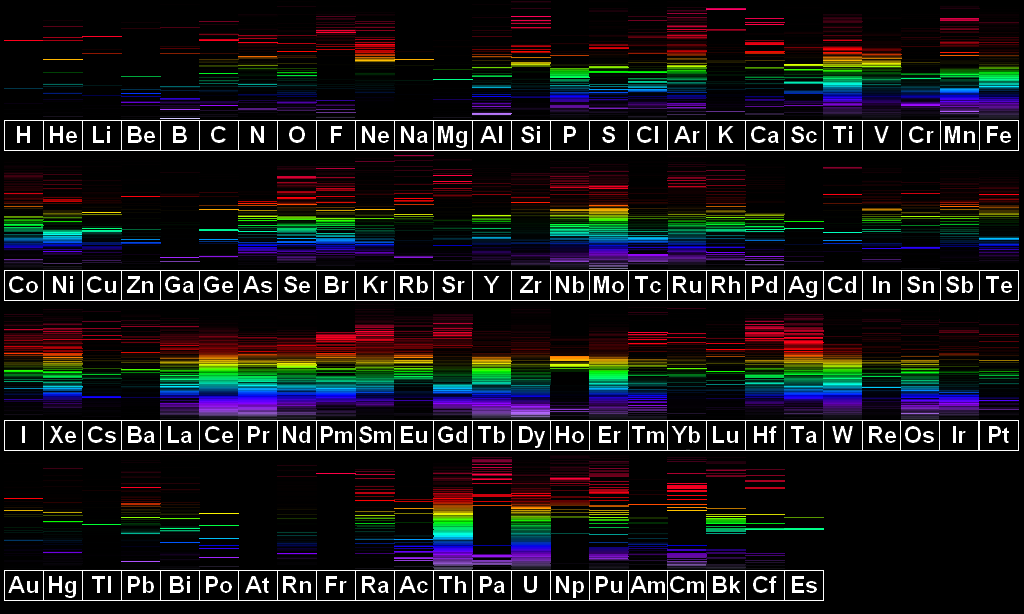
 High Resolution Version |
Periodic table format |
 Interactive Format |

 High Resolution Version |
Periodic table format |
 Interactive Format |
This image is based on spectrum line positions and intensities from MIT Wavelength Tables and the NIST Atomic Spectrum Database. A few spectra (neon, for instance) have had corrections applied in order to more closely match my own personal observations.
Astatine is missing from this image as no visible lines of this element are known. Only one line is shown for francium (7177Å) as that is also the only known line.
Disclaimer: I personally have not had opportunity to observe all elements, especially those which are expensive, hard to find, or radioactive. Therefore, some spectra may deviate considerably from the depictions pictured here. Elements which I have observed in a semi pure state include: hydrogen, helium, nitrogen, neon, sodium, magnesium, aluminum, argon, iron, nickel, copper, zinc, silver, cadmium, tin, xenon, platinum, and mercury. I can vouch for all of these appearing almost exactly as depicted. Elements I have seen in mixtures with other elements include scandium, krypton, molybdenum, and lead. I am reasonably sure that all of these appear more or less as depicted.
The element spectrum images on this page, including the linked high resolution version and the periodic table format image, are Public Domain or CC0.
| Send me an email |